Selinexor is currently the only XPO1 inhibitor approved for commercial use by the FDA and has been extensively tested in clinical trials across numerous cancer indications worldwide since 2012. The proposed clinical trial to treat hospitalized patients with COVID-19 would be the first study of an XPO1 inhibitor in patients with severe viral infections.
“While Karyopharm’s clinical development strategy until now has been focused on patients with various types of cancer, there is increasing evidence that XPO1 inhibition could play an important role in the treatment of patients with viral infections including SARS-CoV-2,” said
“Given the globally devastating impact of the COVID-19 pandemic, innovative strategies and collaborative efforts are critically needed to bring effective treatment options to patients, who are so desperately in need. I am highly encouraged by the scientific rationale of studying selinexor, which targets both virus and immune-mediated injury, for treatment of patients with severe COVID-19. My staff, colleagues, and I and look forward to working with Karyopharm to better understand the role of this novel approach in improving patient outcomes of COVID19,” said
SINE XPO1 inhibitors have demonstrated activity against over 20 different viruses, including the RNA viruses, influenza, respiratory syncytial virus (RSV) and other common causes of respiratory infection. XPO1 inhibition has been identified in several assays as having potential activity against SARS-CoV-2, although specific animal models have not been available to date. One of the most important aspects of COVID-19 is the marked pulmonary inflammation with high levels of cytokines such as IL6, IL1, IFNg and others. Along these lines, selinexor and other SINE compounds have demonstrated potent anti-inflammatory activity through the inhibition of Nuclear Factor kB (NF-kB), leading to reductions in all of these cytokines in a variety of models, and this may be particularly beneficial to hospitalized patients with COVID-19.
Karyopharm’s clinical program in COVID-19 is not expected to impact the timing or prioritization of other key Company milestones, including the planned submission of a sNDA for XPOVIO (selinexor) in combination with once-weekly Velcade® (bortezomib) and low-dose dexamethasone as a new second line treatment for patients with relapsed or refractory multiple myeloma (based on the
About COVID-19 and the Potential Role of XPO1 Inhibition
The novel coronavirus SARS-CoV-2 that emerged from the
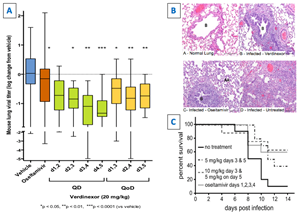
Severe influenza infection shares many of the features of SARS-CoV infection. Verdinexor demonstrated potent inhibition of influenza viral replication, even when given four days post-infection (Figure 1).11,12 In addition, verdinexor induced reductions in markers of disease pathology and improved survival in influenza models in mice and ferrets (Figure 1).11,12
Figure 1: In a mouse H1N1 influenza model, delayed (up to 4 days) verdinexor treatment: (A) significantly reduces influenza virus levels; (B) ameliorated lung injury; and (C) improved survival is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ea80c453-8192-4bea-b8f8-74f7ff5670d2
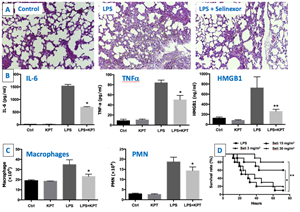
XPO1 is also responsible for the nuclear export and functional inactivation of several of the major anti-inflammatory, antioxidant and cytoprotective transcription factors including IkB, PPARg, RXRa, HMGB1 and Nrf2.13 Conversely, blockade of XPO1 leads to nuclear retention and functional activation of these critical proteins. High levels of XPO1 are found in multiple inflammatory conditions and may amplify ongoing inflammatory responses leading to severe organ, including pulmonary, damage.14 In a mouse model for sepsis, selinexor treatment increased survival following a lethal dose of endotoxin (Figure 2).15 Figure 2: In a lipopolysaccharide (LPS)-induced sepsis model, selinexor (A) attenuated lung injury, (B) reduced serum cytokines levels, (C) reduced macrophage and polymorphonuclear (PMN) subpopulations in the peritoneal exudate and improved survival is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/964c3643-480c-4cc0-a7df-5b2165502349 |
|||||
In addition, selinexor reduced inflammatory cytokine secretion including TNFa, IL-6 and IFNg while reducing the numbers of macrophage and polymorphonuclear neutrophils in the mice peritoneal cavity (the site of the LPS injection).15 Importantly, treatment with selinexor attenuated the acute respiratory distress syndrome (ARDS)-like lung injury observed in this model. Selinexor therapeutic effects were achieved through the inhibition of the inflammatory NFkB pathway, induction of the anti-inflammatory effects of the DNA binding protein HMGB1 by inducing its nuclear retention and reductions in cytokine levels including TNF-a and IL-6.15 These findings are significant as COVID-19 disease severity including respiratory symptoms correlates with the patient’s cytokine levels. Severe disease is characterized by increased interleukin (IL)-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α.16 Moreover, predictors of fatality of 150 confirmed COVID-19 cases in
Together, these results suggest that SINE compounds such as selinexor could provide both anti-viral and anti-inflammatory activities in patients suffering from severe COVID-19 and therefore warrants clinical investigation.
Conference Call Information
Karyopharm will host a conference call today,
About XPOVIO® (selinexor)
XPOVIO is a first-in-class, oral Selective Inhibitor of Nuclear Export (SINE) compound. XPOVIO functions by selectively binding to and inhibiting the nuclear export protein exportin 1 (XPO1, also called CRM1). XPOVIO blocks the nuclear export of tumor suppressor, growth regulatory and anti-inflammatory proteins, leading to accumulation of these proteins in the nucleus and enhancing their anti-cancer activity in the cell. The forced nuclear retention of these proteins can counteract a multitude of the oncogenic pathways that, unchecked, allow cancer cells with severe DNA damage to continue to grow and divide in an unrestrained fashion. Karyopharm received accelerated
For more information about Karyopharm’s products or clinical trials, please contact the Medical Information department at:
Tel: +1 (888) 209-9326
Email: medicalinformation@karyopharm.com
IMPORTANT SAFETY INFORMATION
Thrombocytopenia
XPOVIO can cause thrombocytopenia, leading to potentially fatal hemorrhage. Thrombocytopenia was reported as an adverse reaction in 74% of patients, and severe (Grade 3-4) thrombocytopenia occurred in 61% of patients treated with XPOVIO. The median time to onset of the first event was 22 days. Bleeding occurred in 23% of patients with thrombocytopenia, clinically significant bleeding occurred in 5% of patients with thrombocytopenia and fatal hemorrhage occurred in <1% of patients.
Monitor platelet counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment. Institute platelet transfusion and/or other treatments as clinically indicated. Monitor patients for signs and symptoms of bleeding and evaluate promptly. Interrupt and/or reduce dose, or permanently discontinue based on severity of adverse reaction.
Neutropenia
XPOVIO can cause neutropenia, potentially increasing the risk of infection. Neutropenia was reported as an adverse reaction in 34% of patients, and severe (Grade 3-4) neutropenia occurred in 21% of patients treated with XPOVIO. The median time to onset of the first event was 25 days. Febrile neutropenia was reported in 3% of patients.
Obtain neutrophil counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment. Monitor patients for signs and symptoms of concomitant infection and evaluate promptly. Consider supportive measures including antimicrobials for signs of infection and use of growth factors (e.g., G-CSF). Interrupt and/or reduce dose, or permanently discontinue based on severity of adverse reaction.
Gastrointestinal Toxicity
Gastrointestinal toxicities occurred in patients treated with XPOVIO.
Nausea/Vomiting
Nausea was reported as an adverse reaction in 72% of patients, and Grade 3 nausea occurred in 9% of patients treated with XPOVIO. The median time to onset of the first nausea event was 3 days.
Vomiting was reported in 41% of patients, and Grade 3 vomiting occurred in 4% of patients treated with XPOVIO. The median time to onset of the first vomiting event was 5 days.
Provide prophylactic 5-HT3 antagonists and/or other anti-nausea agents, prior to and during treatment with XPOVIO. Manage nausea/vomiting by dose interruption, reduction, and/or discontinuation. Administer intravenous fluids and replace electrolytes to prevent dehydration in patients at risk. Use additional anti-nausea medications as clinically indicated.
Diarrhea
Diarrhea was reported as an adverse reaction in 44% of patients, and Grade 3 diarrhea occurred in 6% of patients treated with XPOVIO. The median time to onset of diarrhea was 15 days.
Manage diarrhea by dose modifications and/or standard anti-diarrheal agents; administer intravenous fluids to prevent dehydration in patients at risk.
Anorexia/Weight Loss
Anorexia was reported as an adverse reaction in 53% of patients, and Grade 3 anorexia occurred in 5% of patients treated with XPOVIO. The median time to onset of anorexia was 8 days.
Weight loss was reported as an adverse reaction in 47% of patients, and Grade 3 weight loss occurred in 1% of patients treated with XPOVIO. The median time to onset of weight loss was 15 days.
Monitor patient weight at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment. Manage anorexia and weight loss with dose modifications, appetite stimulants, and nutritional support.
Hyponatremia
XPOVIO can cause hyponatremia; 39% of patients treated with XPOVIO experienced hyponatremia, 22% of patients experienced Grade 3 or 4 hyponatremia. The median time to onset of the first event was 8 days.
Monitor sodium level at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment. Correct sodium levels for concurrent hyperglycemia (serum glucose >150 mg/dL) and high serum paraprotein levels. Treat hyponatremia per clinical guidelines (intravenous saline and/or salt tablets), including dietary review. Interrupt and/or reduce dose, or permanently discontinue based on severity of adverse reaction.
Infections
In patients receiving XPOVIO, 52% of patients experienced any grade of infection. Upper respiratory tract infection of any grade occurred in 21%, pneumonia in 13%, and sepsis in 6% of patients. Grade ≥3 infections were reported in 25% of patients, and deaths resulting from an infection occurred in 4% of patients. The most commonly reported Grade ≥3 infections were pneumonia in 9% of patients, followed by sepsis in 6%. The median time to onset was 54 days for pneumonia and 42 days for sepsis. Most infections were not associated with neutropenia and were caused by non-opportunistic organisms.
Neurological Toxicity
Neurological toxicities occurred in patients treated with XPOVIO.
Neurological adverse reactions including dizziness, syncope, depressed level of consciousness, and mental status changes (including delirium and confusional state) occurred in 30% of patients, and severe events (Grade 3-4) occurred in 9% of patients treated with XPOVIO. Median time to the first event was 15 days.
Optimize hydration status, hemoglobin level, and concomitant medications to avoid exacerbating dizziness or mental status changes.
Embryo-Fetal Toxicity
Based on data from animal studies and its mechanism of action, XPOVIO can cause fetal harm when administered to a pregnant woman. Selinexor administration to pregnant animals during organogenesis resulted in structural abnormalities and alterations to growth at exposures below those occurring clinically at the recommended dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with a female partner of reproductive potential to use effective contraception during treatment with XPOVIO and for 1 week after the last dose.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥20%) are thrombocytopenia, fatigue, nausea, anemia, decreased appetite, decreased weight, diarrhea, vomiting, hyponatremia, neutropenia, leukopenia, constipation, dyspnea, and upper respiratory tract infection.
The treatment discontinuation rate due to adverse reactions was 27%; 53% of patients had a reduction in the XPOVIO dose, and 65.3% had the dose of XPOVIO interrupted. The most frequent adverse reactions requiring permanent discontinuation in 4% or greater of patients who received XPOVIO included fatigue, nausea, and thrombocytopenia. The rate of fatal adverse reactions was 8.9%.
Please see XPOVIO Full Prescribing Information available at www.XPOVIO.com.
References
1 Widman, D. et al. In vitro toxicity and efficacy of verdinexor, an exportin 1 inhibitor, on opportunistic viruses affecting immunocompromised individuals. PLoS ONE. 2018. 13(10): e0200043. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0200043
2 Gordon, D. et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug Repurposing. bioRxiv. 2020. 03.22.002386. https://doi.org/10.1101/2020.03.22.002386
3 Zhu, N. et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in
4 Freundt, E. et al. Molecular Determinants for Subcellular Localization of the Severe Acute Respiratory Syndrome Coronavirus Open Reading Frame 3b Protein.
5 Sharma, K. et al. SARS-CoV 9b Protein Diffuses into Nucleus, Undergoes Active Crm1 Mediated Nucleocytoplasmic Export and Triggers Apoptosis When Retained in the Nucleus. PLoS ONE. 2011. 6(5): e19436. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0019436
6 McBride, R. et al. The Coronavirus Nucleocapsid Is a Multifunctional Protein. Viruses. 2014. 6. https://www.mdpi.com/1999-4915/6/8/2991
7 Ujike, M. et al. Two palmitylated cysteine residues of the severe acute respiratory syndrome coronavirus spike (S) protein are critical for S incorporation into virus-like particles, but not for M–S co-localization.
8 Sims, A. et al. Release of Severe Acute Respiratory Syndrome Coronavirus Nuclear Import Block Enhances Host Transcription in Human Lung Cells.
9 Ongoing research being conducted at
10 Zhou, Y. et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery. 2020. 6:14. https://doi.org/10.1038/s41421-020-0153-3
11 Tamir S, et al. Anti-influenza and anti-inflammatory activity of KPT-335, a Selective Inhibitor of Nuclear Export (SINE), in mice and ferrets. Poster presented at: The 27th
12
13 Tajiri, N. et al. A Nuclear Attack on Traumatic Brain Injury: Sequestration of Cell Death in the Nucleus. CNS Neurosci Ther. 2016. https://doi.org/10.1111/cns.12501
14 Perwitasari, O. et al. Verdinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces Influenza A Virus Replication In Vitro and In Vivo. Journal of Virology. 2014. 88:17. https://jvi.asm.org/content/88/17/10228
15 Wu, M. et al. KPT-330, a potent and selective CRM1 inhibitor, exhibits anti-Inflammation effects and protection against sepsis.
16 Huang, C. et. al. Clinical features of patients infected with 2019 novel coronavirus in
17 Ruan, Q. et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from
About
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding Karyopharm’s expectations and plans relating to selinexor as a potential treatment for hospitalized patients with severe COVID-19; the initiation and design of a global randomized clinical trial to study this potential application of selinexor, including the dosing regimen; submissions to, and the review and potential approval of selinexor in this indication by, regulatory authorities, including the anticipated availability of data to support such submissions, timing of such submissions and actions by regulatory authorities and the potential availability of accelerated approval pathways; and the therapeutic potential of and potential clinical development plans for Karyopharm’s drug candidates, including the impact of a selinexor clinical trial on the timing or prioritization of other key company milestones, such as its expected submission of a supplemental new drug application in the second quarter of 2020 for XPOVIO in combination with once-weekly Velcade® and low dose dexamethasone. Such statements are subject to numerous important factors, risks and uncertainties, many of which are beyond Karyopharm's control, that may cause actual events or results to differ materially from Karyopharm's current expectations. For example, there can be no guarantee that Karyopharm will successfully complete necessary clinical development phases of selinexor in this indication; that data from a clinical trial of selinexor would support its use in treatment of hospitalized patients with severe COVID-19; that regulators will approve the use of selinexor in hospitalized patients with severe COVID-19, or that such approval will be made on an accelerated timeline. Further, there can be no guarantee that any positive developments in the development or commercialization of Karyopharm’s drug candidate portfolio will result in stock price appreciation. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the risk that the COVID-19 pandemic could disrupt Karyopharm’s business more severely than it currently anticipates, including by reducing sales of XPOVIO, interrupting or delaying research and development efforts, impacting the ability to procure sufficient supply for the development and commercialization of selinexor or other product candidates, delaying ongoing or planned clinical trials, impeding the execution of business plans, planned regulatory milestones and timelines, or inconveniencing patients; the adoption of selinexor for treatment of COVID-19 in the commercial marketplace, the timing and costs involved in commercializing selinexor for such indication or any of Karyopharm’s drug candidates that receive regulatory approval; the ability to retain regulatory approval of selinexor for such indication or any of Karyopharm’s drug candidates that receive regulatory approval; Karyopharm's results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the
Velcade® is a registered trademark of Takeda Pharmaceutical Company Limited.
Contacts:
Investors:
857-297-2241 | ikarp@karyopharm.com
Media:
FTI Consulting
Simona Kormanikova or Robert Stanislaro
212-850-5600 | Simona.Kormanikova@fticonsulting.com or robert.stanislaro@fticonsulting.com
Source: Karyopharm Therapeutics Inc.